A COVID-19 booster targeting two versions of the coronavirus in one shot offered stronger and broader protection than the current booster, which targets only one version, according to clinical trial results released this week by vaccine maker Moderna.
The results are preliminary and have not been peer reviewed or published in a scientific journal. But Moderna touted the findings as evidence that bivalent or multivalent vaccines—those that target two or more versions of the virus in a single shot—are the way forward for COVID-19 boosters.
Moderna and other vaccine makers are on a mission to develop boosters that could restore the once extraordinarily high levels of protection that mRNA-based COVID-19 vaccines initially provided, while also protecting against future variants. The first-generation mRNA vaccines were all designed to target the ancestral version of SARS-CoV-2 isolated in Wuhan, China—and they did so quite effectively, showing efficacy against symptomatic disease in the ballpark of 95 percent. But the virus has evolved into variants that can evade vaccine-derived protections. The latest variant, omicron, significantly reduced vaccine effectiveness against symptomatic disease, though protection against severe disease remains strong. Booster doses of the current vaccine design buoy protection but don’t restore the high levels seen previously. And the virus continues to evolve.
As such, vaccine makers are testing variant-specific boosters as well as combination shots. Moderna and Pfizer/BioNTech—joint makers of the other leading mRNA COVID-19 vaccine—swiftly announced plans for an omicron-specific vaccine in December, before the fast-moving variant swept the globe. But so far, early animal data has suggested that a booster dose targeting only the omicron variant may not offer an advantage over the current vaccines at protecting against omicron. While variant-specific vaccine trial data continues to come in, vaccine makers have also been working on combination shots. Earlier this month, for instance, the National Institutes of Health announced the start of a clinical trial (in collaboration with Moderna) that is testing six different booster regimens, four of which involve a combination shot.
Fresh data
Moderna now has data on one of its first combination shots, which targets the ancestral strain plus the beta variant. The beta variant was first identified in South Africa and dubbed a “variant of concern” in December 2020 after it demonstrated an ability to evade vaccine-derived immune responses. Though experts initially feared it would cause an omicron-like wave of infections, beta never became widely prevalent in the US and has since been completely elbowed out by omicron.
Still, vaccine makers had begun working on beta-targeting vaccines last year. And that work has proven somewhat useful now because beta shares some of the key mutations for dodging protective antibodies that are found in omicron. Thus, combination vaccines targeting beta may foreshadow advantages that omicron-targeting combination vaccines may have in the future.
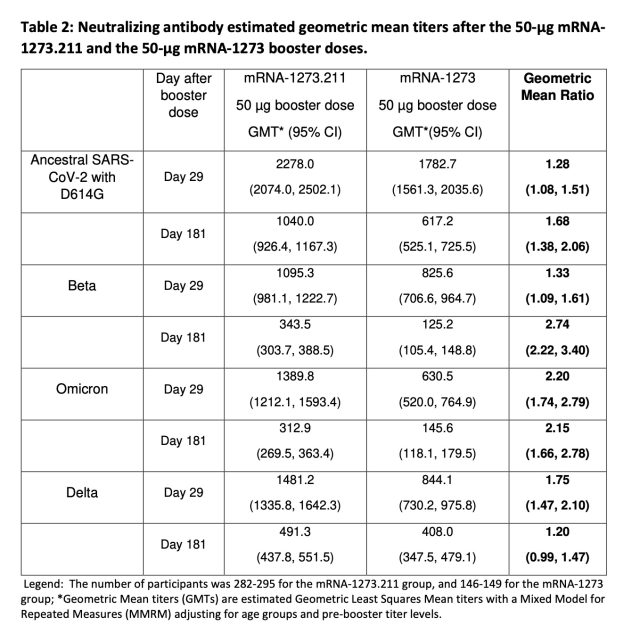
The fresh data released by Moderna looked at neutralizing antibody levels in around 300 people who received a 50-microgram dose of the beta/ancestral combo vaccine (dubbed mRNA-1273.211). Antibody levels in that group were compared with those from around 150 people who received the current 50-microgram booster (mRNA-1273) that targets the ancestral version of the virus. Compared with the current booster, the beta/ancestral combo shot generated higher levels of neutralizing antibodies against the ancestral virus as well as the beta, omicron, and delta variants. In the case of omicron, the combo shot generated neutralizing antibody levels twice as high as the current shot (when comparing geometric mean titres). That two-fold advantage was maintained after six months, as well. Further, there were no safety concerns with the combo vaccine during the trial and the side-effect profile looked about the same as that of the current booster.
Fall strategy
Of course, this study has limitations. The number of people in the trial was not huge, and the study is not large enough to look at vaccine effectiveness. The study also does not look at other types of immune responses, such as cell-based responses. But it did strongly suggest that a bivalent vaccine could out-compete the current vaccine, because neutralizing antibody levels tend to correlate with protection. The authors of the study speculated that the additional virus targets present in the combination vaccine induce “further maturation and evolution” of antibody responses in vaccinated people. “Therefore, immunization with the primary series does not set a ceiling to the neutralizing antibody response,” they wrote, “and a booster dose of the bivalent vaccine elicits a robust response with titers that are likely to be protective against COVID-19.”
In a statement, Moderna CEO Stéphane Bancel said the findings have bolstered the company’s optimism for combination shots. “We believe that these results validate our bivalent strategy, which we announced and began pursuing in February 2021. The results indicate that mRNA-1273.211 [the combo shot] at the 50 µg dose level induced higher antibody responses than the 50 µg mRNA-1273 [current] booster, even when additional variants of concern were not included in the booster vaccine,” Bancel said.
With their strategy set for future boosters, Moderna expects to provide newly formulated boosters for the fall. However, Bancel anticipates that an omicron/ancestral combo booster will provide even stronger, broader protection.
“Our latest bivalent booster candidate, mRNA-1273.214, which combines the currently authorized Moderna COVID-19 booster with our omicron-specific booster candidate, remains our lead candidate for the fall 2022 Northern Hemisphere booster,” Bancel said. “We look forward to sharing initial data on mRNA-1273.214 later in the second quarter. We believe that a bivalent booster vaccine, if authorized, would create a new tool as we continue to respond to emerging variants.”