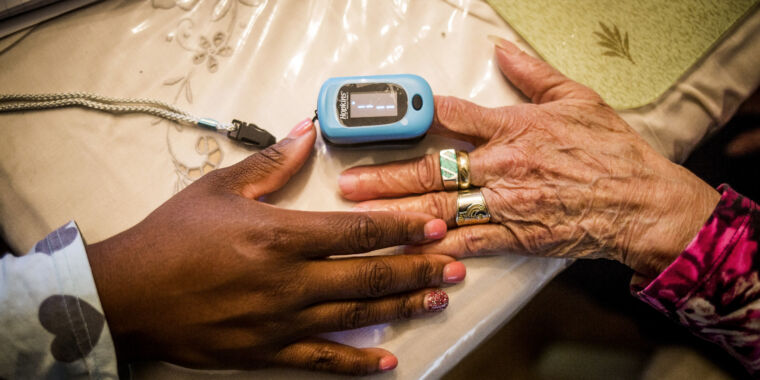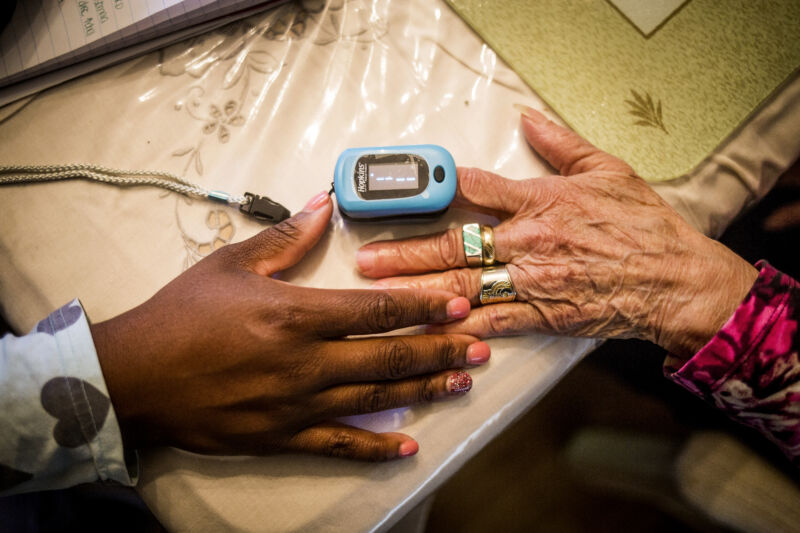
For years, studies have found racial bias in common oxygen measuring devices called pulse oximeters, as well as alarming dangers from inaccurate blood oxygen measurements in dark-skinned patients. Now, the US Food and Drug Administration is summoning its expert advisors to review the problematic devices and consider new recommendations and regulatory actions.
The FDA announced Thursday that its advisory committee—the Anesthesiology and Respiratory Therapy Devices Panel (ARTDP)—would convene on November 1 to discuss pulse oximeters. Until then, the agency renewed emphasis on the safety warning it issued in February 2021, which noted that the ubiquitous devices “may be less accurate in people with dark skin pigmentation.”
That warning closely followed a study from December 2020 that highlighted the racial bias of pulse oximeters amid the COVID-19 pandemic. The global spread of a respiratory disease with a hallmark symptom of breathing difficulty sent pulse oximeter usage soaring—elevating the problem of racial disparities. The 2020 study—led by researchers in Michigan and published in the New England Journal of Medicine—found that pulse oximeters were nearly three times more likely to miss dangerously low blood oxygen levels (hypoxemia) in Black patients compared with white patients.
From there, several other studies corroborated the racial bias and highlighted the danger it posed to dark-skinned patients during the pandemic and beyond. But, it certainly wasn’t the first study to report the concerning bias. Researchers have long noted the racial disparity, with studies dating as early as 1991.
Dubious devices
Pulse oximeters were developed in the 1970 and have since become a mainstay in routine patient care, with current devices typically clipping onto a finger. They estimate blood oxygen saturation (SpO2) by assessing the relative absorbance of two wavelengths of light (red and infrared, generally) beamed into the finger, plus the pulse-based flow of blood through the arteries.
But, the devices were mainly tested and calibrated on light-skinned patients. Researchers suspect that the high levels of skin pigment, melanin, in dark-skinned patients can interfere with the absorbance measurements. Numerous studies have found that pulse oximeters tend to overestimate oxygen saturation in dark-skinned patients.
The dangers of those faulty readings were realized during the pandemic. A study published in May found that pulse oximeter’s overestimation of SpO2 in Black and Hispanic patients with COVID-19 caused significant delays in care, including access to lifesaving treatments, such as dexamethasone. For some patients, the faulty readings meant their eligibility for treatment was never recognized by the devices. That study, led by researchers at Johns Hopkins University, appeared in JAMA Internal Medicine.
In July, another study in JAMA Internal Medicine by researchers in Boston, found darker skinned patients in intensive care who had inaccurate pulse oximeter readings ended up receiving less supplemental oxygen. Meanwhile, a study published in the same month in BMJ by researchers in Michigan looked at records of more than 30,000 patients at the Veterans Health Administration between 2013 and 2019. It found that Black patients were more likely to have hypoxemia undetected by pulse oximetry. The study notes that hidden hypoxemia is linked to an increased risk of morbidity and mortality.