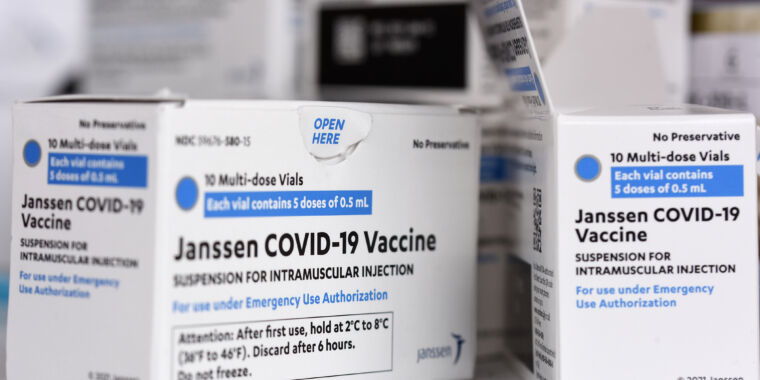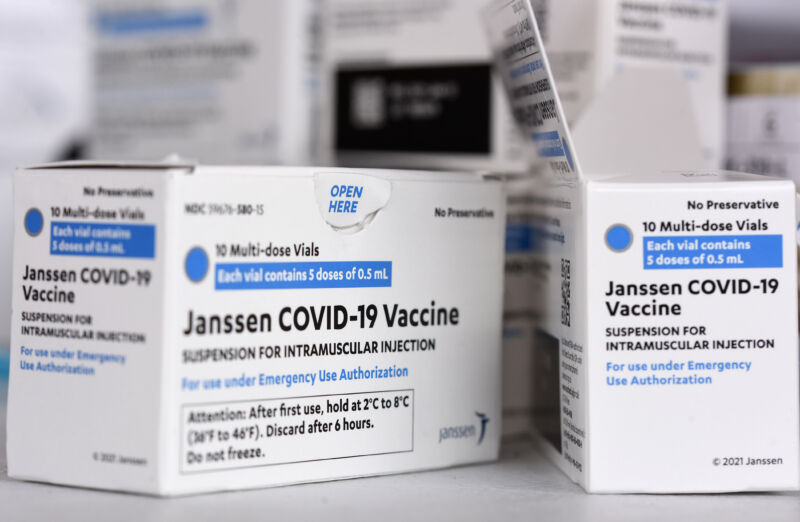
The US Food and Drug Administration limited the use of the Johnson & Johnson (Janssen) COVID-19 vaccine late Thursday, citing the risk of a very rare but severe clotting disorder called thrombosis with thrombocytopenia syndrome (TTS).
From now on, the J&J vaccine is only to be used in people ages 18 and up who are unable or unwilling to receive an alternative COVID-19 vaccine. That includes people who have had a life-threatening allergic reaction (anaphylaxis) to an mRNA COVID-19 vaccine, people who have personal concerns about mRNA COVID-19 vaccines and would otherwise not get vaccinated, and people who don’t have access to mRNA COVID-19 vaccines.
The limitation comes as the FDA and the Centers for Disease Control and Prevention have been closely monitoring people who received J&J COVID-19 vaccinations for TTS. To date, the agencies have identified and confirmed 60 cases of TTS linked to the vaccine, including nine deaths. That represents a rate of 3.23 TTS cases per million doses of J&J vaccine administered, and a rate of 0.48 TTS deaths per million doses of vaccine administered, the FDA said Thursday.
Though the risks are small, the FDA determined that they’re unnecessary risks for most people to take, given the wide availability of mRNA vaccines (made by Moderna and Pfizer-BioNTech) that are similarly effective and do not carry such risks of severe disease and death.
The FDA’s decision follows a downgraded recommendation from the Centers for Disease Control and Prevention last December, which stated that the mRNA COVID-19 vaccines are preferred over the J&J vaccine. The CDC outlined specific instances in which the J&J vaccine could be considered, which match the uses listed by the FDA.
Limits and risks
In a statement Thursday, top vaccine regulator Peter Marks explained the timing of the FDA’s move. “We recognize that the Janssen COVID-19 vaccine still has a role in the current pandemic response in the United States and across the global community. Our action reflects our updated analysis of the risk of TTS following administration of this vaccine and limits the use of the vaccine to certain individuals,” he said, and “demonstrates the robustness of our safety surveillance systems and our commitment to ensuring that science and data guide our decisions. … The agency will continue to monitor the safety of the Janssen COVID-19 Vaccine and all other vaccines, and as has been the case throughout the pandemic, will thoroughly evaluate new safety information.”
TTS is a severe condition marked by the unusual combination of blood clots blocking a blood vessel, aka thrombosis, and thrombocytopenia, an overall low count of blood platelets, which help blood clot. The condition can be particularly dangerous if the blood clot affects the brain, such as in cerebral venous sinus thrombosis (CVST), which is a rare but life-threatening type of stroke that prevents blood from draining out of the brain.
The risk of TTS from the J&J vaccine—which uses an adenovirus-based vaccine design—was first identified in early April 2021, at which point the CDC paused use of the vaccine. The FDA and CDC lifted the pause later that month after determining that the vaccine’s benefits in preventing COVID-19 outweighed the small risk of developing TTS. It still remains unclear how the vaccine may trigger the condition in rare instances, however researchers hypothesized that something about the adenovirus-based vaccine may trigger an immune response that leads to platelet activation and low platelet levels. Another adenovirus-based COVID-19 vaccine, made by AstraZeneca, has also been linked to rare cases of TTS.
Amid the TTS reports, the CDC’s pause, and early clinical trial data showing that mRNA vaccines outperformed the J&J vaccine, use of the troubled adenovirus-based vaccine plummeted in the US. Of the 577 million doses administered to date, only 18.7 million were J&J vaccines.