A committee of experts advising the Food and Drug Administration voiced unanimous support Wednesday for the authorization of two COVID-19 vaccines for children under the age of 5. If the FDA authorizes the vaccines, it will mark the first time during the more than two-year pandemic that vaccines against COVID-19 will be available for this age group—the last group yet to be eligible for vaccination.
Although children in this young age group have a relatively lower risk of severe disease and death from COVID-19 compared with older groups, they can and do become severely ill and die from the infection. As of last month, 45,000 children under 5 have been hospitalized for COVID-19 during the pandemic; roughly 50 percent of those hospitalizations occurred during the omicron wave. Of the children who land in the hospital, about 63 percent have no underlying medical conditions that put them at greater risk of severe COVID-19. And about a quarter of those hospitalized require intensive care.
So far, 475 children under the age of 5 have died from COVID-19 during the pandemic, making COVID-19 far deadlier than other diseases we routinely vaccinate young children against, including influenza, measles, chickenpox, hepatitis A, and rotavirus.
“I think we have to be careful that we don’t become numb to the number of pediatric deaths because of the overwhelming number of older deaths,” Peter Marks, FDA’s top vaccine regulator, said today. “Every life is important.”
Marks made his comments at the start of today’s full-day gathering of the FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC). The independent experts reviewed data on Moderna’s vaccine for infants 6 months old to children under the age of 6 years and Pfizer-BioNTech’s vaccine for infants 6 months to children under 5 years.
The committee voted unanimously—21 to 0—in support of the FDA authorizing Moderna’s vaccine for infants and young children. In a second vote, they also voted unanimously—21 to 0—in support of the FDA authorizing the Pfizer-BioNTech vaccine for infants and young children.
These votes are the first steps in a four-step process for getting these vaccines into tiny arms. The second step is for the FDA to decide whether to issue authorizations for both vaccines. The agency isn’t obligated to follow VRBPAC’s advice but typically does, and it is widely expected to do so here, likely by the end of Thursday.
Process
The third step is for the Centers for Disease Control and Prevention to convene its advisory committee of independent vaccine experts, the Advisory Committee on Immunization Practices (ACIP), which will review the data again and vote whether to recommend the vaccines’ use. The ACIP is scheduled to meet to discuss and vote on these vaccines Friday, June 17, and Saturday, June 18. If ACIP members vote in favor of the CDC recommending the use of these vaccines, then the fourth and final step will be for CDC Director Rochelle Walensky’s endorsement. At that point, administration can begin.
In terms of distribution of the vaccines, the Biden administration has already put up a supply of 10 million doses—some Moderna doses and some Pfizer-BioNTech doses—for states to pre-order. If or when the FDA authorizes the vaccines, then those orders can be shipped to states. If all goes to plan, orders of doses are expected to arrive in states this weekend. Assuming Walensky endorses the use of the vaccines, federal officials expect vaccine administration to begin “in earnest” starting Tuesday, June 21—given that Monday is a federal holiday observing Juneteenth.
In the meantime, parents eager to finally get their young children protection against the devastating pandemic virus will have to carefully determine which of the two vaccine options is suitable for their kids. Both vaccines are simply smaller doses of the companies’ mRNA-based vaccines already approved for older age groups. But, compared with each other, they have key differences in dosing, timing of doses, efficacy estimates, and side effects.
While VRBPAC members were overwhelmingly supportive of authorizing both vaccines, they also expressed reservations over the Pfizer-BioNTech vaccine’s longer three-dose, three-month-long regimen, and extremely preliminary vaccine estimates.
Here’s a rundown of the two vaccines, the data we have on them, and what VRBPAC members thought.
Doses: Two doses, each 25 micrograms, a quarter of the adult primary-series doses.
Timing of doses: Four weeks total. The second dose is given 28 days after the first.
Trial size:
Moderna looked at safety, immune responses, and efficacy in a randomized, placebo-controlled trial containing 4,048 children ages 2 to 5 years (3,040 were vaccinated, 1,008 got placebo), and 2,355 infants ages 6 months to 23 months (1,762 were vaccinated, 593 got placebo).
Immune responses:
Both age groups met the trial’s primary endpoint of generating equivalent antibody levels to those seen in adults ages 18 to 25 years.
Efficacy estimates:
The efficacy estimates were determined in the omicron era and, thus, were lower than earlier trial data with previous variants.
The efficacy against symptomatic COVID-19 for children 6 to 23 months ranged from 31.5 percent to 50.6 percent, depending on how the cases were defined. Moderna used two definitions: the CDC case definition, which defines a case as having one systemic or one respiratory symptom plus a positive RT-PCR; or its own”301 case definition,” which is defined as having two systemic or one respiratory symptom plus a positive RT-PCR.
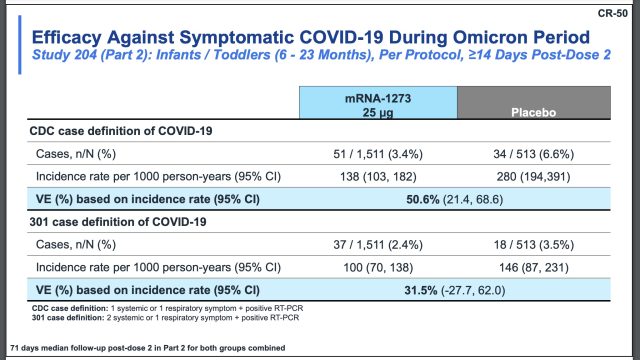
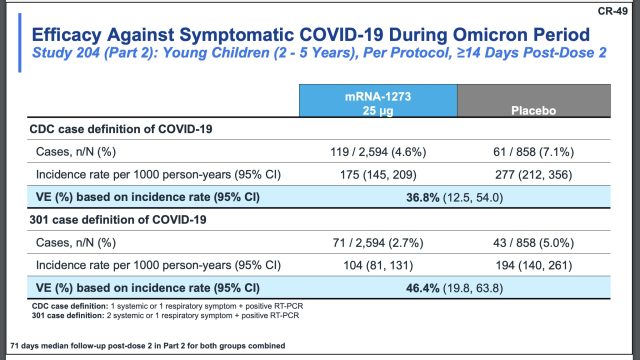
For children ages 2 to 5 years, the efficacy estimates range from 36.8 percent (CDC definition) to 46.4 percent (301 case definition).
Safety:
The overall safety profile looked comparable to other childhood vaccines, with some children developing fevers and fatigue, and infants reporting irritability, and loss of appetite. The majority of the fevers were considered on the low end (100.4°F to 102.0°F), with 0.4 percent of toddlers and 0.2 percent of babies developing fevers over 104°F. Most of the fevers occurred within two days of vaccination, and the median duration of the fever was a day.
One 17-month-old girl developed a fever and had a seizure possibly linked to the vaccine. She also developed a rash the next day, and it’s unclear what caused it. The seizure occurred after the first vaccine dose. There were few other specifics on the child’s case, but Moderna representatives noted that the child appeared to recover well and went on to get the second dose. Fever-related convulsions, called febrile seizures, occur in up to 5 percent of children under the age of 5 years and can be triggered by various illnesses. The vast majority are harmless and do not cause long-term damage, though they are frightening for parents.
There are no cases of myocarditis, MIS-C, or death in the trial.
Final thoughts:
Overall, the committee was generally pleased with the data. “I think the evidence is pretty clear… this vaccine is very effective; it’s also very safe to use,” Jay Portnoy, a pediatrician at Children’s Mercy Hospital in Kansas City and VRBPAC member, said. “I’m a little disappointed that it doesn’t prevent infection by COVID as effectively as it could, because that’s what spreads it around… but at least we can stop people from being terribly sick.”
After the vote, Portnoy added: “This will certainly alleviate a lot of [parents’] concerns, and so I’m really happy the vote went the way it did. I think it was the right vote.”
Doses: Three doses, each 3 micrograms, a tenth of the adult dose.
Timing of doses: 11 weeks total. The second dose is given 21 days after the first, and the third dose is given at least eight weeks after the second.
That said, In the clinical trial, toddlers and infants received the third dose up to 20 weeks after the second. Generally, immune responses tend to be stronger with longer intervals between doses.
Trial size:
Pfizer-BioNTech looked at safety, immune responses, and efficacy in a randomized, placebo-controlled trial containing 4,562 children ages 6 months to under 5 years (3,013 were vaccinated, 1,513 got placebos). Immune responses: The children met the trial’s primary endpoint of generating equivalent antibody levels to those seen in adults ages 16 to 25 years—after the third dose.
Two doses were not enough to generate high enough antibody responses in children ages 2 to 5 years, the companies previously reported.
Efficacy estimates:
Pfizer and BioNTech did not reach the pre-set endpoint of having 21 cases in their trial, which was the point at which efficacy estimates would be calculated. Instead, they had 10 cases: seven in the placebo group and three in the vaccinated group.
The case numbers are small, and the follow-up time was also a concern. While Moderna followed up on trial participants for a median of over two months, Pfizer had median follow-up times of five to six weeks.
Still, Pfizer and BioNTech presented preliminary efficacy estimates that are impressively large, but have enormous confidence intervals.
Overall, Pfizer and BioNTech reported that their vaccine was 80.3 percent effective against symptomatic COVID-19 during the omicron era in children 6 months to under 5 years. That broke down to 82 percent effective for children 2 to 5 years, and 75.5 percent for infants 6 months to under 2 years. But again, the confidence intervals are very wide, particularly for the younger age group, where the interval ranged from -370 to 99.6. Negative numbers are never good to see on confidence intervals, but such as large negative number does not inspire confidence.
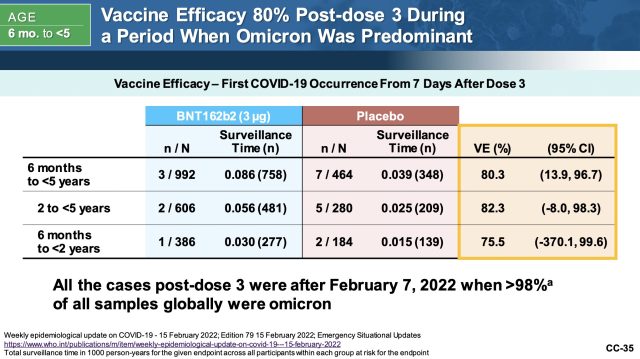
The data is “limited by a small number of cases and a limited follow-up time,” Doran Fink, the FDA’s acting deputy director of the Office of Vaccines Research and Review, said in the meeting today. “We do consider this estimate to be preliminary, we consider it to be imprecise and potentially unstable, and so exactly what the vaccine efficacy is after dose three, I think, needs further data.”
-
Efficacy estimates over time for children 6 months to 23 months
-
Efficacy estimates for children ages 2 to 4 years
After the vote, several VRBPAC members expressed concern that the high efficacy estimates could get promoted without that key context, making the Pfizer-BioNTech vaccine look far superior to Moderna’s without the crucial caveats of the estimates’ significant weaknesses. “We really can’t go with the point estimates [of efficacy] because the confidence intervals for a lot of them are pretty wide,” VRBPAC Chair Arnold Monto said in response to some of the concern.
-
Efficacy in children 6 months to 23 months. Note
-
Efficacy for children ages 2 to 4 years
Pfizer and BioNTech also looked at efficacy over time for the vaccine, which essentially showed that two doses offered very little to no protection against COVID-19. This too, worried committee members.
Safety:
With the much lower vaccine dose, the safety profile for the Pfizer vaccines looked good. Reports of adverse events were very similar to placebo.
Final thoughts:
Voting that the benefits of this vaccine outweigh the risks “is something I can support,” said Paul Offit, an infectious disease expert at The Children’s Hospital of Philadelphia. “But I do have some concerns about this vaccine… It does worry me actually that there was no protection after dose two. That was surprising, I think it was probably surprising to the company. I fear that they may have under-dosed.”
The immune response data is reassuring after dose three, Offit noted, but needing three doses is a concern. “So for people who’ve gotten that vaccine, who’ve gotten, say, two doses of that vaccine, they have to know they’re not protected, and they’re going to have to wait a few months until they are protected. And I just wonder whether parents will understand that,” he said. “I do worry about this because I think it was a surprisingly negative result.”








