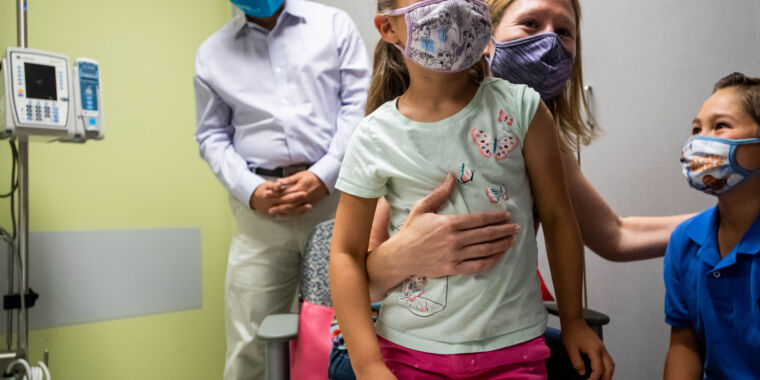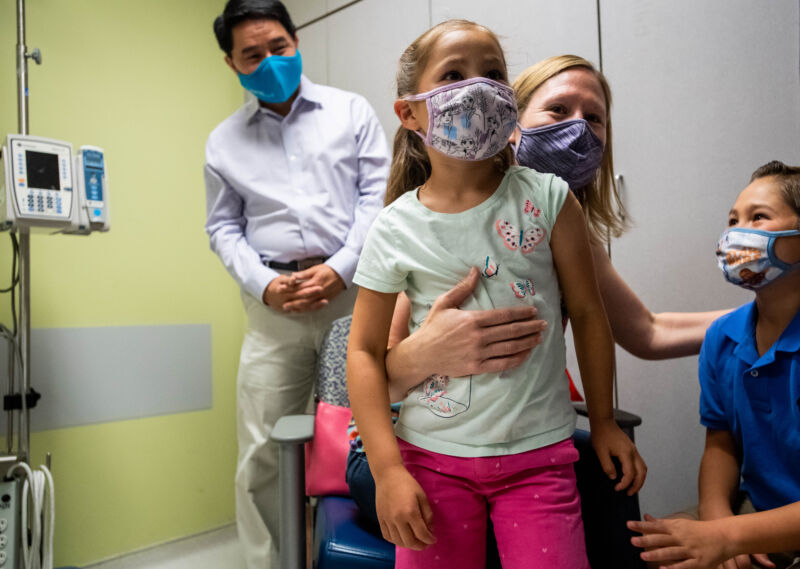
Wednesday brought some potentially positive news for the parents and caregivers of young children who have endured an agonizing wait for an effective COVID-19 vaccine. Moderna announced Wednesday that its two-dose vaccine for children ages 6 months to under 6 years appeared safe and produced strong antibody levels that correlate with effectiveness in adults. The company plans to ask the Food and Drug Administration to authorize the vaccine in the coming weeks.
The trial, a randomized, observer-blind, placebo-controlled study called KidCOVE, involved 6,700 children under 6 years old (4,200 children six months to 2 years and 2,500 children 2 years to under 6 years). Vaccinated children received two 25-microgram doses of vaccine—a quarter of the adult dose—which were given 28 days apart. Neutralizing antibody levels in the vaccinated children met or exceeded those seen in adults ages 18 to 25, for which vaccine is already approved.
Omicron hit
Though the primary objective of the trial was to reach those antibody levels seen in adults—a process called an immunobridging study—the trial also looked at efficacy against infection and severe disease amid the wave of omicron coronavirus variant infections. Phase III trial data indicated that the vaccine was about 44 percent effective at preventing an omicron infection in children ages 6 months to 2 years and 37.5 percent effective against an omicron infection in children ages 2 years to under 6 years.
“The secondary endpoint of vaccine efficacy confirms statistically significant but lower efficacy against COVID-19 infection as expected during the omicron wave and consistent with adult observational data,” Moderna reported. The company did not provide case numbers or other efficacy data. But it did note that it defined statistical significance as having a 95-percent confidence interval with a lower bound of anything greater than zero, hinting that there may be wide intervals and case numbers too small to calculate more accurate efficacy estimates.
The company reported that most COVID-19 cases in the trial were mild, and none of the children in the trial (vaccinated or unvaccinated) developed severe disease, were hospitalized, or died. As such, Moderna was unable to assess efficacy against those severe outcomes.
As for safety, the profile of the children’s vaccine looked a lot like what has been seen in adults. Most of the side effects were mild to moderate, and they were more common after the second dose. The rate of fevers greater than 38°C (100.4°F) was similar to what is seen in other pediatric vaccines. In the study, 17 percent of children ages 6 months to 2 years and about 15 percent of children 2 years to under 6 years reported fevers. Only a few children (0.2 percent in each age group) reported fevers above 40°C (104°F). There were no reports of myocarditis or pericarditis (inflammation of the heart and surrounding tissue) or multisystem inflammatory syndrome in children (MIS-C).
“We believe these latest results from the KidCOVE study are good news for parents of children under 6 years of age. We now have clinical data on the performance of our vaccine from infants six months of age through older adults,” Moderna CEO Stéphane Bancel said in a statement. “Given the need for a vaccine against COVID-19 in infants and young children, we are working with the US FDA and regulators globally to submit these data as soon as possible.”
Rollercoaster
The company added that it expected to file those submissions in the “coming weeks.” It also announced that it has started the submission process for a two-dose vaccine for children ages 6 years to under 12, for which the company had previously reported results. The vaccine is already approved for that age group in Australia, Canada, and Europe.
Moderna’s news could come as a welcome relief to parents and caregivers of children six months to under six years. Though the vaccine efficacy estimates with potentially wide confidence intervals are not ideal, the doses may offer baseline protection from severe outcomes, which could be critical for higher-risk children. Parents and caregivers have experienced an agonizing wait for any protection for their youngest during the pandemic—and they’ve endured a rollercoaster ride of updates from Pfizer and BioNTech on the progress of their pediatric doses.
In December, the companies announced that their two-dose vaccine did not meet the primary goal of their immunobridging study, producing relatively weak antibody levels in youngsters aged 2 years to 5 years. The companies then said they would add a third dose to the trial in an effort to boost those levels.
Then the omicron wave came in January, and it hit young children harder than previous waves of the pandemic. With more COVID-19 case data in their trial, Pfizer and BioNTech announced in late January that they would, after all, try to move forward with just two doses of their vaccine. But that plan was scuppered again a few weeks later when the companies and the FDA backtracked. They collectively announced that based on undisclosed trial data, they had decided to wait for data from the third doses once again. The companies are now expecting those results next month, which would put the earliest availability of their vaccine sometime in May if all goes well.