On Wednesday night, Elon Musk hosted an update from his brain-computer interface company, Neuralink. Most of the update involved various researchers at the company providing overviews of the specific areas of technology development they were working on. But there wasn’t anything dramatically new in the tech compared to the company’s 2020 update, and it was difficult to piece the presentations together into a coherent picture of what the company plans to do with its hardware.
But probably the most striking thing is that the prior update indicated that Neuralink was getting close to human testing. Over a year later, those tests remain about six months out, according to Musk.
Lots of tech
Neuralink involves a large series of overlapping technical efforts. The interface itself requires electrodes implanted into the brain. To connect those electrodes with the outside world, Neuralink is using a small bit of hardware implanted in the skull. This contains a battery that can be recharged wirelessly, and a low-power chip that gathers data from the electrodes, performs some simple processing on it, and then transmits that data wirelessly.
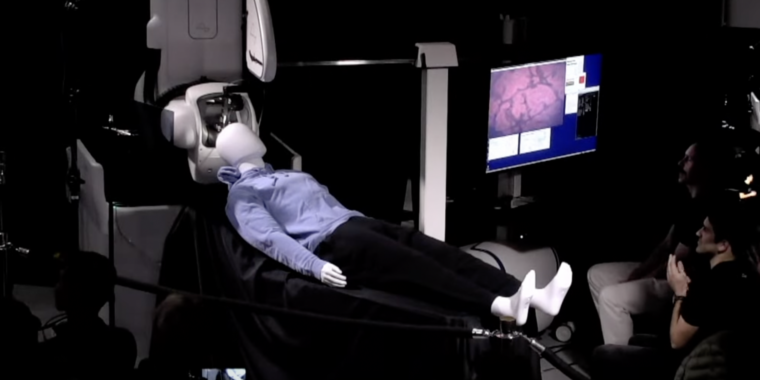
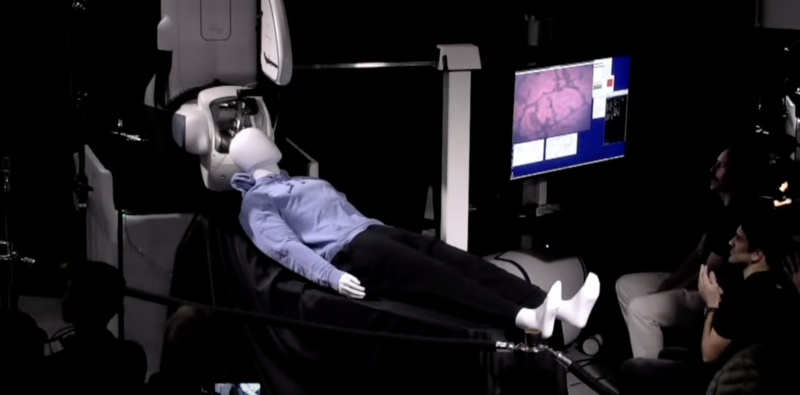
Getting all that in place requires delicate neurosurgery, and the company is developing a surgical robot to make that process safe and consistent.
On the other end of the process, neural signals have to be interpreted in near real time to understand what’s happening in a given brain region. This requires computer systems that can handle everything from patient-to-patient variability to hour-to-hour differences in brain activity. Finally, in some cases, the device will need to send information back to the brain in a way that the nerve cells there can interpret (either immediately or following a learning process).
That’s… a lot of things. And the event saw people talking about almost all of them. In many cases, the information was substantially similar to what was shown the year before. Various animals with implants were shown doing everything from playing Pong to manipulating cursors and typing using their implants—more examples than last year, but not radically different. Similarly, Musk talked a bit more about the implant’s processing capacity, now provided partly by an ARM processor. There are some indications of evolutionary progress, but there are no indications that it’s close to a finalized design that’s ready for a Food and Drug Administration submission.
Perhaps the most significant difference from years prior is the level of detail involved in the surgical robot. This time, there was both an on-stage demo of the hardware and a fair bit of time spent discussing the details of the surgical procedure it was being developed for. In the previous update, the development of the robot appeared to be lagging.
We’ve been here before
The event was said to be a general overview of the company’s activities, and the presentations seemed to cover all of the key areas Neuralink is working on. But there are issues with that approach.
One is that brain implants have been an active research area for decades. While the details are different, many things Neuralink was showing off have been done before. To an extent, that’s understandable. Neuralink is developing its own electrodes, implant, and processing system. As such, it needs to demonstrate that these systems can perform like previously tested electrodes in animal experiments. But, so far, at least, Neuralink hasn’t provided any indications that its systems are superior to those that have already been tested extensively or were on a trajectory to get there.
Meanwhile, some of its competitors progressed in the areas where Neuralink sought to differentiate itself. Blackrock Neurotech, for example, is now touting fully implantable electronics that offer wireless charging and data transfer. And the company has already sent hardware through a clinical trial and is applying for FDA approval. In fact, the company has several additional clinical trials in progress.
The custom surgical robot seems unique to Neuralink (though surgical robots are widely used for other purposes). But one of the Neuralink staff mentioned that the robot was a sticking point with the FDA, saying it’s difficult to demonstrate its safety to the satisfaction of regulators. And another one of its competitors, Synchron, hopes to avoid the need for major surgery by using blood vessels to get implants deep into the brain. And those devices have also managed to go through clinical trials already.
Another problem with Neuralink’s progress update is that it doesn’t clearly indicate that the company is ready to go to the FDA. Starting a clinical trial will mean that the company has finalized a hardware design (even if it’s working on next-generation hardware separately) and chosen a specific neural defect that it plans to treat. The update’s scattershot progress reports gave no indication that any of that has been done.
None of this is to say that there won’t ultimately be space for multiple technologies in the brain-computer implant space. Neuralink will likely eventually arrive where some of these other companies are now, or it might find a niche where its hardware is especially effective. But so far, the company isn’t sharing any information that indicates that it’s close to either result—much less accomplishing some of the more outlandish claims thrown around by Musk.
Neuralink’s presentation is available online. Oddly, for an organization run by a self-professed fan of free speech, the company has disabled comments on the video.