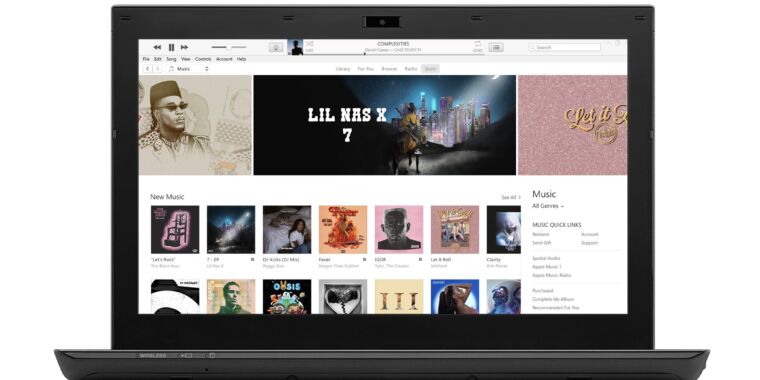
Slow-motion video of boiling ice, a research project of the Nature-Inspired Fluids and Interfaces Lab at Virginia Tech.
Dash a few drops of water onto a very hot, sizzling skillet and they’ll levitate, sliding around the pan with wild abandon. Physicists at Virginia Tech have discovered that this can also be achieved by placing a thin, flat disk of ice on a heated aluminum surface, according to a new paper published in the journal Physical Review Fluids. The catch: there’s a much higher critical temperature that must be achieved before the ice disk will levitate.
As we’ve reported previously, in 1756, a German scientist named Johann Gottlob Leidenfrost reported his observation of the unusual phenomenon. Normally, he noted, water splashed onto a very hot pan sizzles and evaporates very quickly. But if the pan’s temperature is well above water’s boiling point, “gleaming drops resembling quicksilver” will form and will skitter across the surface. It’s called the “Leidenfrost effect” in his honor.
In the ensuing 250 years, physicists came up with a viable explanation for why this occurs. If the surface is at least 400 degrees Fahrenheit (well above the boiling point of water), cushions of water vapor, or steam, form underneath them, keeping them levitated. The Leidenfrost effect also works with other liquids, including oils and alcohol, but the temperature at which it manifests will be different.
The phenomenon continues to fascinate physicists. For instance, in 2018, French physicists discovered that the drops aren’t just riding along on a cushion of steam; as long as they are not too big, they also propel themselves. That’s because of an imbalance in the fluid flow inside the Leidenfrost drops, acting like a small internal motor. Large drops showed a balanced flow, but as the drops evaporated, becoming smaller (about half a millimeter in diameter) and more spherical, an imbalance of forces developed. This caused the drops to roll like a wheel, helped along by a kind of “ratchet” effect from a downward tilt in the same direction the fluid in the droplet flowed. The French physicists dubbed their discovery a “Leidenfrost wheel.”
In 2019, an international team of scientists finally identified the source of the accompanying cracking sound Leidenfrost reported. The scientists found that it depends on the size of the droplet. Smaller drops will skitter off the surface and evaporate, while larger drops explode with that telltale crack. The culprit is particle contaminants, present in almost any liquid. Larger drops will start out with a higher concentration of contaminants, and that concentration increases as the droplets shrink. They end up with such a high concentration that the particles slowly form a kind of shell around the droplet. That shell interferes with the vapor cushion holding the drop aloft, and it explodes when it hits the surface.
And last year, MIT scientists determined why the droplets are propelled across a heated oily surface 100 times faster than on bare metal. Under the right conditions, a thin coating formed outside each droplet, like a cloak. As the droplet got hotter, minuscule bubbles of water vapor began to form between the droplet and the oil, then moved away. Subsequent bubbles typically formed near the same spots, forming a single vapor trail that served to push the droplet in a preferred direction.
But can you achieve the Leidenfrost effect with ice? That’s what the Virginia Tech team set out to discover. “There are so many papers out there about levitating liquid, we wanted to ask the question about levitating ice,” said co-author Jonathan Boreyko. “It started as a curiosity project. What drove our research was the question of whether or not it was possible to have a three-phase Leidenfrost effect with solid, liquid, and vapor.”